

 |
 |
Salix atrocinerea and related willows in eastern MassachusettsAlexey Zinovjev and Irina Kadis
Abstract The European willow Salix atrocinerea
introduced to eastern North America is considered a distinct species—and
not a subspecies of S. cinerea.
Diagnostic characters distinguishing S. atrocinerea
from related willows occurring in eastern Massachusetts are discussed.
S. cinerea is present in Massachusetts along with
S. atrocinerea, though it is
less common. More evidence is needed in order to justify the inclusion
of other willows from the same section, S. caprea and S. aurita,
on the list of European plants naturalized in New England.
Some of these
records might be based on S. atrocinerea (or
S. cinerea) hybrids with the native S. bebbiana or S. discolor.
A possibility
of hybridization between the alien S. atrocinerea
and native S. humilis is demonstrated through
obtaining hybrid seedlings from S. humilis seeds
collected in the Boston area. S. atrocinerea is
known to be invasive in some countries outside the United States. In New
England, its hybridization with the native willows may create a threat
for their existence in addition to displacement. S. bebbiana appears to have been eliminated from the vicinity of
Boston, the area invaded
by S. atrocinerea.
Introduction
Willows are usually considered a difficult group, many species
treated differently by different authors. This is true for the Atlantic
West-European species Salix atrocinerea Brotero, or
rusty willow, naturally growing in Britain, France, Belgium, Spain,
Portugal, on some Mediterranean islands, and in North Africa. It has been
considered a subspecies of the European/West-Siberian gray willow:
S. cinerea ssp. oleifolia
(Smith) Macreight (Meikle 1984, Berg 2000, Haines 2008, and others).
However, many contemporary authors, including the world authorities on the
genus Salix, treat rusty and gray willow as two different species (Argus
1997, 2005, 2007; Chmelar, Meusel 1986; Rechinger 1993; Skvortsov
1968, 1999; etc.). Remarkably, phenetic analysis of a Spanish population
of S. atrocinerea demonstrated that it clusters with
S. aurita L., S. starkeana
Willd., and S. bebbiana Sarg.—and not with
S. cinerea L. This supported the species rank for
rusty willow (Argus 1997: 76, footnote).
The fact that rusty willow was included with gray willow has
produced confusion. While gray willow is well known to have been
introduced from Europe to many countries, rusty willow has not been
distinguished in the majority of cases. It is highly probable that some
records of gray willow actually refer to rusty willow. For example, in
Australia and New Zealand gray willow is considered to be a highly
invasive plant. The presence of rusty willow in Australia was directly
confirmed only recently by Cremer (2003), who listed both subspecies of
S. cinerea.
While S. cinerea had been long known as an
introduced species in North America, S. atrocinerea
was first identified in 1985 (Argus 1985, 1986) as herbarium specimens
from North Carolina, the oldest of which had been collected in 1929 and
1939. Recently rusty willow was found in New York, most of New England,
and southern Canada (Tucker 1996, 2006, 2007; Rawinski 2005a, 2005b; Argus
2005, 2007). In fact, we have found this willow to be the most conspicuous in
eastern Massachusetts and particularly around Boston (Rawinski 2005a,
2005b; Kadis 2007; Zinovjev, Kadis 2008). We found rusty willow common in
nearly all of eastern Massachusetts counties: Essex, Middlesex, Norfolk,
Suffolk, Bristol, Plymouth, and Barnstable—i.e., everywhere where we
made observations.
We also observed it in central Massachusetts (Worcester
County), where it appears to be more rare. In addition to examining
willows in nature, we had an opportunity to study some recent willow
collections from Worcester County provided by Robert Bertin as well as
eastern Massachusetts samples in the Harvard University Herbaria.
Our observations of S. atrocinerea have been restricted to
only New England populations. Apparently, the variability range in the
introduced S. atrocinerea cannot be the same as in
the area of its natural distribution in Atlantic Europe. Yet the only
specimen of S. atrocinerea we observed in Europe
looked quite similar to American plants. (It was a young cultivated shrub
in a private willow collection north of its natural range, in southern
Finland.)
Despite its prominence, the alien willow has been overlooked for
decades, taken for either some native willows, mostly S. bebbiana or S. discolor Muhl., or else for
other alien willows (S. caprea L. or S. cinerea). Apparently, we still don't have knowledge of the
entire area rusty willow has acquired in eastern North America. Most
likely, it is still being overlooked in some parts of the Atlantic Coast,
particularly between New York and North Carolina.
S. atrocinerea is rather closely related to
those American and European willows with which it has been confused,
nearly all of them belonging to the same large group: sect.
Cinerella (Argus 1997, 2007) or sect.
Vetrix, subsect. Laeves
(Skvortsov 1968, 1999). The only exception is S. bebbiana, which is related somewhat more distantly. It has been
placed in sect. Fulvae (Argus 1997) or in a different
subsection (subsect. Substriatae) of section
Vetrix (Skvortsov 1968, 1999). One important
morphological difference between S. bebbiana and the
rest of the willows in question is the bud gradation type. Skvortsov
(1968, 1999) distinguished three generic types of bud gradation along the
shoot in willows and named them alba-,
arctica-, and caprea-type (fig.
1-2). While willows belonging
to Cinerella are all characterized by the
caprea-type of bud gradation (fig.
2), in S. bebbiana buds exhibit a different pattern and rather approach
the alba-type (fig. 1).
It was some ten years ago that we tentatively named a willow from
the Boston area S. atrocinerea for the first time. We
had not been familiar with S. atrocinerea in Europe;
besides, the overall morphological variability of willow populations
around Boston was so high that segregating willows into species appeared
not as simple as in European Russia or Finland. Separating S. atrocinerea from the native S. bebbiana
was particularly difficult. The latter is supposed to be one of the most
common all across Massachusetts; however, it turned up to be impossible
to find a typical Bebb willow in the Boston area. Those specimens we
originally named S. bebbiana never looked exactly as
the S. bebbiana we had observed in upstate New York,
Canada, and different parts of Eurasia. It was much later that we found
typical S. bebbiana in western and central
Massachusetts. Yet we never identified a single typical S. bebbiana in the east, though plenty of old samples from eastern
Massachusetts exist in herbarium collections. The presence of strange
specimens reminding of Bebb willow along with complete absence of typical
S. bebbiana can be explained if we assume that this
willow has been eliminated around Boston due to hybridization with an
alien species.
Willows are known to easily produce hybrids, though frequency of
hybrids is often over-estimated. For example, many authors claim frequent
hybrids between the common European willows S. caprea, S. cinerea, and S. aurita. However, according to Buser (1940:
653-655) and Skvortsov (1968, 1999: 72), hybrids between these species
either do not exist or at least are extremely rare. Our own observations
in northern Europe fully support this opinion. Abundant 'hybrids' found in
nature often are nothing but misunderstood normal species.
Skvortsov (1968, 1999), agreeing with Wichura (1865) and Buser (1887, 1940), stated that
hybridization becomes significant only in specific habitats—either
naturally unstable or disturbed by humans; yet even in those areas that
are comparatively hybrid rich, hybrids usually don't dominate over parent
species.
However, when alien willows
are introduced outside their native range due to human activities, the
geographic isolation of species ceases to exist. Thus previously isolated
species come into direct contact without having developed isolation
mechanisms that prevent hybridization in nature. Most of the time
adventive species occupy disturbed habitats known to constitute
hybrid-rich zones. An exceptionally high rate of hybrid formation in these
areas would not be normally possible in nature.
The situation with S. atrocinerea in New England appears to present
one of such cases of abnormal prolific hybrid formation.
We are going to address
the problem of hybrid formation with alien species in a different
publication. Here we are going to demonstrate the existence of hybrids
between S. atrocinerea and one more native willow
from sect. Cinerella, S. humilis
Marsh., by observing the seedlings originating from wild-collected
S. humilis seeds.
Salix atrocinerea: morphological characters
and comparison with related willows
Willows from the sect. Cinerella present a
particular challenge when it comes to identification of dry samples; it is
much more effective to look at live specimens. Winter is the best time to
observe such important diagnostic characters as bud shape and position.
Bark and wood surface characteristics are also available in winter (fig.
3-30). Observations of aments in spring are important by all means; yet
the best time for willow identification is late summer and fall—the time
when both leaves and buds are fully developed. Here is a brief account of
some important characters in rusty willow, as compared to those of closely
related species.
Rusty willow, if it is not damaged, grows as a tree
to 10-12 m tall
(fig. 53,
56),
even though much more often we have seen it growing as a
multi-stem tree/shrub
(fig. 52)
or even just a shrub (fig. 51).
Of New England related
willows, only S. bebbiana can grow as a tree.
(The rest of tree willows in New England—S. nigra Marshall,
S. alba L.,
S. fragilis L., and hybrids of S. babylonica L.—do not belong to the same subgenus and look quite different.)
Tree
growth is also characteristic of S. caprea; however,
the latter has hardly ever become naturalized in New England. All the
three species, differ from each other by the bark (fig. 21-30).
Prominent, dense and long longitudinal ridges (striae) are formed on
S. atrocinerea wood underneath the bark (fig. 17). The wood ridges correspond to
longitudinal depressions on the bark surface. The smooth, light gray bark on mature trunks and
limbs is thus characteristically 'wavy' (fig. 21-22). Ridged wood is a character shared with
S. cinerea, but that species does not grow as a tree.
Besides, in European S. cinerea we never observed
such prominently wavy bark as in populations of S. atrocinerea in Massachusetts. In S. caprea
and S. bebbiana, wood ridges are either completely
absent or short and sparse; the corresponding 'waves' on the bark are never formed. The
bark on limbs and younger trunks of S. caprea is
smooth, frequently quite distinctly greenish; its surface not wavy (fig.
23-25). The bark of mature S. bebbiana
is very
peculiar (fig. 28-30): with a beautiful 'braided'
pattern, so that mature specimens of S. bebbiana can
hardly be confused with other willows.
S. atrocinerea produces buds of two contrasting
types, groups of large floriferous buds found in-between small vegetative
buds. Skvortsov (1968, 1999) called this bud pattern
caprea-type (fig. 2). It is typical for most species
of the sect. Cinerella. This character helps separate
S. atrocinerea from more distantly related
S. bebbiana, whose floriferous buds are hardly
distinguishable from vegetative (alba-type of bud
gradation, fig. 1).
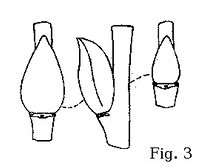
Floriferous buds in rusty willow are usually positioned at an angle
to the branchlet, as the adaxial bud surface is convex; the lateral carina typically forms a sigmoidal curve when the bud is viewed
from the side (fig. 7-9). This bud shape makes S. atrocinerea similar to the European related species (fig.
3-5) but different from all the related
willows native to New England (fig. 12-14).
Branchlets in S. atrocinerea are typically
slender, glabrous, reddish, though these characters are considerably variable. In this character,
S. atrocinerea resembles the native S. bebbiana as well as European S. aurita;
this should help distinguish S. atrocinerea from the
native S. discolor and introduced S. cinerea with much thicker branchlets and branches. In
S. cinerea branchlets also differ in color—they
are entirely gray, dull 'ash' (hence the name gray willow or ash willow
for the latter species).
Aments (fig. 37-38) in S. atrocinerea are short-stalked, densely flowered, their rachises
never exposed. Bracts are black. The aments can hardly be confused with
those of S. bebbiana, which are borne on foliated
stalks, become very loose when ripe, and have light-colored bracts. (fig.
39).
In S. atrocinerea leaf venation (up to the
third order) is prominent on the lower leaf surface forming reticulum
(fig. 35) and a
corresponding faint rugose pattern on the upper surface. This character is
shared by nearly all of the discussed species including S. bebbiana. However, this makes S. atrocinerea different from S. discolor,
which does not have reticulum on the lower leaf surface (fig. 36).
Rusty willow has received its common name for the ferrugineous
trichomes that are often present on its lower (and sometimes upper) leaf
surface together with grayish trichomes. While the presence of these rusty
trichomes is diagnostic, their absence does not necessarily exclude
S. atrocinerea. Ferrugineous trichomes may be more or
less abundant or completely absent. Ferrugineous trichomes may be also
found in S. discolor, but not in S. bebbiana, neither S. cinerea.
In S. atrocinerea the upper leaf surface is
bright green and shining (fig. 31). S. cinerea
leaves (as well as its gray branchlets), in contrast, are
characteristically dull (fig. 32).
Stipules in S. atrocinerea are typical for most
of the willows from Cinerella: broad, distinctly
inequilateral, which helps distinguish S. atrocinerea
from S. humilis.
As to the leaf shape, in rusty willow it is highly variable. Even
though for a trained eye the variability range is somewhat different from
that of other species, it is difficult to describe the difference
concisely.
Salix atrocinerea and S. cinerea
S. atrocinerea has Mediterranean and
South-European Atlantic distribution type, while S. cinerea is distributed in Eurasia, east and somewhat north of
S. atrocinerea range. Therefore, it is only natural
that S. atrocinerea is much more common in eastern
Massachusetts, on the Atlantic Coast than the continental S. cinerea. In eastern Massachusetts we saw just some few willows that could
be reliably named S. cinerea. Even though it is
sometimes difficult to separate dry herbarium samples, in nature the two
willows typically look rather different from each other.
S. cinerea can be normally distinguished from
S. atrocinerea as follows. This is a multi-stem shrub to
5-6(7) m, never growing as a tree; its branchlets stout; branches, branchlets, and buds grayish, with
dense persistent pubescence (fig. 10-11); leaves with
maximal width distinctly above the middle, gradually attenuate toward
base; upper leaf surface rather dull, lower surface with grayish
trichomes; ferrugineous trichomes completely absent.
Salix bebbiana
Bebb willow, S. bebbiana Sargent
(S. starkeana ssp. cinerascens Hultén) is nearly
circumboreal. It is distributed across the entire North America and all of
Siberia, reaching west to Scandinavia. On a large part of the European
territory S. bebbiana is replaced by vicarious
S. starkeana Willd. (Both can be as well treated as
subspecies of a single species.) Within New England, we saw the typical
S. bebbiana
only on territories less infested
by
S. atrocinerea, such as western Massachusetts or New
Hampshire; however, we have recently examined it in Colorado and earlier
in New York State, Canada, different parts of Siberia, and northern
Finland.
Bebb willow is either a shrub or a tree up to 10 m. Pistillate
specimens of S. bebbiana can be readily separated
from related species in New England at the end of the flowering season,
when their characteristic aments with light-colored bracts become
particularly loose due to growth of ovary stipes.
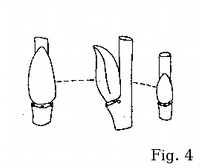
Bebb willow may be also easily distinguished from rusty willow by
the bud shape and pattern: its floriferous buds aren't very different from
vegetative (cf. fig. 6).
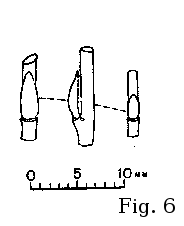
Argus (2005) has classified S. bebbiana bud pattern
with the alba-type (fig. 1). The buds are much more flat and
elongate than those of S. discolor, S. atrocinerea, S. cinerea, S. caprea, or S. aurita, looking similar to
those of vicarious European S. starkeana (fig. 6).
Mature Bebb willows can be distinguished from all other willows by
their peculiar bark with characteristic 'braided' pattern (fig. 28-30).
[1]
The bark pattern
seems to be a reliable character—at least in those populations
we observed in the US. Bark in specimens from central Massachusetts,
upstate New York, and even Colorado looked quite similar.
The ridges under the bark are
either inconspicuous or completely absent.
Putative hybrids of Salix atrocinerea and
S. bebbiana
The specimens we treat as hybrids between the two willows can be
distinguished even during the winter, as their buds are more or less
similar to those of S. bebbiana (fig. 49-50), while the bark looks much
like that of S. atrocinerea: smooth and wavy, as the
wood ridges are developed much better than in typical S. bebbiana (fig. 18).
We have found the 'braided' bark character in S. bebbiana reliable enough for us to consider 'bebbiana-like' mature
willows with smooth bark as hybrids, though further observations
of putative hybrids, particularly during the time of flowering, are
desirable. So far we only observed willows partially resembling
S. bebbiana in their summer and autumn stage and
never had a follow-up ament observation for any known putative
S. atrocinerea x S. bebbiana
hybrid.
Salix humilis and S. occidentalis
Upland or prairie willow S. humilis Marsh. is
an eastern North American shrub of small to medium size, not more than 3 m
tall.
Though we have found S. humilis to be rather
common in central Massachusetts, we located only a few scattered
individuals in the Boston area: some few in the Middlesex Fells
Reservation north of Boston and only three in the Stony Brook Reservation,
southern Boston. All three solitary individuals in Stony Brook are
rather low and delicate, even resembling dwarf upland willow S. occidentalis Walter (S. tristis
Aiton).
Many authors consider the latter as just a variety of S. humilis: S. humilis var.
tristis (Aiton) Criggs or S. humilis var. microphylla (Anderss.) Fern.
Others treat it as a distinct species (Gleason, Cronquist 1963: 239,
1991: 173; Skvortsov 1971). S. occidentalis is a
delicate shrub only up to 0.7 m with miniature leaves. It is impossible to
confuse this willow with S. atrocinerea. So far we
have found it only twice: in the Middlesex Fells Reservation and Myles
Standish State Forest.
In addition to its habit, S. humilis can be
distinguished from S. atrocinerea by the
following characters. Floriferous buds in S. humilis are oblong-ovoid (fig. 14-15). Leaves are more elongate, with
somewhat revolute margins, their maximum width well above the middle.
Lower leaf surface is densely tomentose, at least in young leaves.
Stipules, when present, are subsymmetrical, nearly lanceolate to
linear (fig. 14). In the latter
character S. humilis (together with S. occidentalis) is different from the rest of the willows
discussed in this article.
Hybrids of Salix atrocinerea with S. humilis
S. atrocinerea is abundant in the Stony Brook Reservation.
Due to the rareness of same-species 'partners'
around, scattered individuals of S. humilis might be
pollinated by the readily available pollen of rusty willow and thus produce hybrid
seed. In order to test this hypothesis, we grew plants from seeds
collected from one of S. humilis. The progeny grew
into vigorous, fast-growing, coarse single-stem plants (fig. 44-45), which apparently have
little to do with a delicate mother shrub (fig. 42-43). One of the two grown plants
(fig. 46) has distinctly
wavy bark characteristic of S. atrocinerea. Both
willows would actually fit S. atrocinerea,
if it were not their elongate leaves (fig. 48),
somewhat decumbent shoots (as in S. humilis or S. occidentalis),
atypical aments (fig. 47),
and floriferous buds of an odd shape (fig. 16).
Salix discolor
The North American pussy willow S. discolor Muhl. is
a boreal, mostly eastern species of wide distribution. This
tall shrub can grow up to 6(8) m. Usually pussy willow can be
distinguished from related species by its leaves, which are rather broad,
lacking impressed veins above and conspicuous reticulation beneath (with
mostly just first-order veins prominent).
Decorticated
wood is mostly smooth or with sparse and short, indistinct ridges (fig.
20).
Bark on old stems may form ridges producing irregular 'braided' pattern
(fig. 26-27).
The floriferous buds are large,
often with black scales during the winter time. The bud shape is
drastically different from that in related European willows, including
S. atrocinerea.
The bud shape in S. discolor is indeed closer to that of willows from other sections, such
as S. hastata L. or even S. phylicifolia L. or S. pulchra Scham.
(Skvortsov 1955: fig. 7;
Belyaeva et al. 2006: fig. 41, 68, 71).
The aments are somewhat similar
to those of S. atrocinerea, especially at the start
of their development. Precocious, appearing very early in spring, they are
very large and dense. However, later on pistillate aments become loose,
though never as lax as those of S. bebbiana
(fig. 39). Another diagnostic character of S. discolor is long, spreading stigmas (fig. 40).
Even though a possibility of its hybridization with S. atrocinerea cannot be excluded (see the notes under
S. caprea), S. discolor remains
relatively common around Boston, sometimes growing together with
S. atrocinerea in wet habitats.
Salix caprea
Goat willow, S. caprea L. is an Eurasian
willow having a habit of a tree—sometimes a mighty tree (Lagerström,
Uronen 2005: 118, photo); otherwise, with a transitional multi-stem
shrub/tree habit. This is one of the most mesophilous and shade-tolerant
species among the European willows, so that it is frequently found in
relatively dry woods, far away from any water (while both S. cinerea and S. atrocinerea are typical
wetland species).
The bark on old goat-willow trunks forms coarse longitudinal
fissures (fig. 23), as in most
tree willows. On younger trunks and branches the bark is smooth, like in
S. atrocinerea, though without any trace of wavy
surface due to complete absence of ridges on the wood underneath the bark.
The bark on younger limbs is greenish-gray (fig. 24-25).
Under the name 'pussy willow,' it has been frequently recorded as
'cultivated' and 'naturalized' in the United States. However, this can
hardly be true, since S. caprea is very difficult to
propagate from cuttings. In most cases, these records are to be attributed
to S. cinerea or S. atrocinerea
(Argus 1986: 115, 120).
On the other hand, some cultivated 'pussy' willows we observed did not
look like S. cinerea or S. atrocinerea. In this respect, a noteworthy remark by A.
Skvortsov comes to mind. While reviewing yet another Old World species
from the same group, S. aegyptiaca L., he described
it as 'favored for cultivation, since it is easily propagated from
cuttings, unlike S. caprea' (Skvortsov 1999: 178).
One may assume that S. aegyptiaca might have been also
overlooked in the United States—taken for S. caprea. As far as we know, there are no records of
wild-collected S. aegyptiaca in North America.
One more possibility is that some hybrid willows have been taken for
goat willow. S. caprea is first and foremost
characterized by large leaves—mostly broader than in the majority of
related species (fig. 33). A
few times we have observed around Boston strange non-native willows with
unusually broad, often suborbicular leaves. In other respects they did not look
anything like S. caprea. We cannot yet tell if these
fit within the variability range of S. atrocinerea or
also represent hybrid specimens. In the latter case, the parent from which
the broad leaves had been inherited could only be S. discolor, as this is the only native species in our area with a
similar leaf length-to-width ratio. These plants of obscure origin might
have been taken for S. caprea.
We haven't had an opportunity to study the S. caprea samples from the southeastern US cited by Argus (1986:
115). So far none of those specimens we observed in Massachusetts seemed
to be true S. caprea.
More evidence is needed in support of the opinion that S. caprea has been naturalized in eastern North America.
Salix aurita
Eared willow, S. aurita L. is another European
species considered to be introduced and even naturalized in two of the
eastern states: Pennsylvania and Massachusetts. However, it is important
to note that, similarly to S. caprea, this willow
cannot be easily propagated from cuttings. According to Belyaeva et al.
(2006), the outcome of propagation from cuttings in S. aurita is less than 5%—nearly as low as in S. caprea (0-2%).
Eared willow has received its name for the characteristic prominent
and persistent stipules, which we have not observed in specimens
identified as S. aurita from Massachusetts. In Europe
S. aurita mostly grows as a comparatively low shrub
only to 1-2 m in height—much lower than S. cinerea. Its tall-growing variety is known in Scandinavia (Berg
2000, Roininen et al. 2001, Vikberg pers. comm.), though even when it
grows tall, its stem never reaches the diameter of S. atrocinerea. In comparison with S. atrocinerea, leaves of S. aurita are more
dull, of somewhat different color, with even more pronounced reticulate
pattern on both sides (fig. 34). Its floriferous buds are
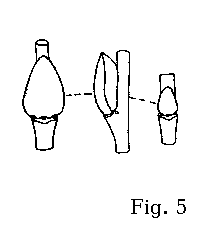
slightly different from those in the rest of related willows (fig. 5). The bracts in S. aurita pistillate aments are paler than in S. atrocinerea and S. cinerea.
So far we don't have evidence which would justify the inclusion
of S. aurita in the flora of North America. A few
herbarium samples from Massachusetts identified as S. aurita we have examined, rather represent S. atrocinerea or, even more likely, its hybrids with S. bebbiana. S. aurita really resembles
S. bebbiana or S. atrocinerea,
so that poorly collected specimens of S. atrocinerea
can be easily mistaken for S. aurita.
Discussion
Generally speaking, willow identification is not as difficult as it
is usually considered. Though it may not be easy to name every specimen,
particularly when hybrids are involved, it should not be difficult to
discriminate between the New England native willows and non-native
S. cinerea and S. atrocinerea
together with their putative hybrids—at least in the field. The
alien willows are readily identifiable even during the winter. The
peculiar smooth, wavy bark of S. atrocinerea and its
hybrids never occurs in any native willows in New England. The sigmoidal
floriferous bud shape typical for European S. atrocinerea, S. cinerea, and S. caprea is different from the bud shape in any related
New England willows.
Even when S. atrocinerea buds lack the typical sigmoidal shape,
they still look very much different from buds of any native willows.
Both S. atrocinerea and S. cinerea start flowering earlier than the majority of native
willows. When a tall staminate S. atrocinerea goes into flower,
it can be spotted from afar. The only native willow around Boston that may
flower at the same time is S. discolor. Though
S. discolor can grow nearly as tall as S. atrocinerea, it has a different habit, usually forming a
spreading rounded shrub, while S. atrocinerea
typically develops a narrow upright crown.
Certainly, upon noticing a staminate S. atrocinerea from the distance,
one needs to take a closer look to confirm the initial identification.
Like many other alien invasive plants, such as glossy buckthorn or
Oriental bittersweet, S. atrocinerea takes advantage
of a prolonged vegetation season, as compared with the growing season of
the native willows. In other words, it once more becomes prominent in the
landscape late in the fall, retaining its characteristic dirty-green
foliage well after all the native willows and the majority of other
native woody plants have dropped their leaves. The leaves often stay on
branches until the first frost and then sometimes remain as marcescent, thus, again, making
the alien willow noticeable at the beginning of winter (fig. 57).
S. atrocinerea and S. cinerea (either in the broad or narrow sense) have not yet been
included on the Invasive Plant List of Massachusetts, despite the evidence
provided by Rawinski (2005a, 2005b, etc.).
S. atrocinerea frequents roadside ditches and other roadside wet
habitats, sometimes penetrating into open wetlands (fig. 51-52).
We have found
S. atrocinerea and its hybrids on nearly every pond
shore around Boston, though only rarely in shaded vernal pools (Stony
Brook Reservation).
According to Rawinski (Cutko, Rawinski 2007: 86), the
invasive willows are not infrequent in vernal pools.
While S. atrocinerea is capable of
vegetative reproduction, the major mechanism of expansion appears to be
seed dispersal. Rusty-willow seedlings and saplings (fig. 54-55) occur around many
ponds, reservoirs, and lakes including habitats of rare pondshore species,
such as Plymouth gentian. Massive germination of rusty willow (on average,
15 seedlings per sq. m for the total plot area of 92 sq. m) was recorded
in the Blue Hills Reservation, at the bottom of the recently drained Blue
Hills Reservoir (2006, own unpublished data). Our impression is that alien
willows are on the rise in eastern Massachusetts, becoming more and more
prominent every year.
Taking into consideration a possibility of hybridization between the
alien and native willows on a large scale, one may predict that the harm
produced by the invasive species in New England
is going to exceed that
inflicted in Australia or New Zealand, where there are no native willows.
In New England, a few native willows might become extinct by way of
producing hybrids with rusty willow (Kadis, Zinovjev 2008).
There already exists a precedent in the human history when an
introduced willow eliminated a native species in Europe by way of forming
hybrids with it. Centuries ago, when S. fragilis L.
was introduced from Asia Minor to Europe, it produced a huge secondary
range there and started to hybridize with the native European willow
S. alba L. on such a grand scale that both pure
S. alba and pure S. fragilis
have become rare within a major part of Europe (Skvortsov 1973). This
hybrid complex has been introduced to the New World under the names of
S. alba and S. fragilis.
Acknowledgments
Tom Rawinski's breakthrough was critical for our understanding of
the alien willow. He was the first person with whom we could share our
initial concerns. We are grateful to George Argus for his continuous
support, confirmation of the identity of the alien willow, and also for completing a
short survey of S. atrocinerea populations in the
Boston area in 2006. We are indebted to Robert
Bertin, Paul Somers, and Tom Palmer for their helpful comments on drafts of
this article. We are grateful to Robert Bertin for showing
S. bebbiana and other willows from Worcester County.
Photography of European willows would not be possible
without generous help of Veli Vikberg (Turenki, Finland). European
rusty willow was observed in Tapani Uronen's private willow collection in Kasiniemi, Padasjoki (Finland).
References
Argus, G.W. 1985. Computerized catalogue of
herbarium specimens of Salix in the southeastern
United States. National Museum of Canada. Ottawa, ON,
Canada.
Argus, G.W. 1986. The genus
Salix (Salicaceae) in the southeastern United States.
Syst. Bot. Monogr. 9. 170 pp.
Argus, G.W. 1997. Infrageneric classification
of Salix (Salicaceae) in the New World. Syst. Bot.
Monogr. 52. 121 pp.
Argus, G.W. 2005. Guide to the genus
Salix (willow) in New England and New York. New York
Flora Association. Salix identification workshop.
June 10-12, 2005. Website http://www.nyflora.org/Workshops/Salix_Workshop/GuideSalixNE-NY4Mar05.pdf
(accessed 16 January 2009).
Argus, G.W. 2007. Salix L.
(Salicaceae) distribution maps and a synopsis of their classification in
North America, north of Mexico. Harvard Papers in Botany 12: 335-368.
Online version: http://aknhp.uaa.alaska.edu/willow/pdfs/Argus_2007_Synopsis_Maps.pdf
(accessed 16 January 2008).
Belyaeva I.V., O.V. Epanchintseva, A.A.
Shatalina, and L.A. Semkina. 2006. Willows of Ural: Atlas and
identification key. UB RAS, Ekaterinburg. 176
pp.
Berg, C.C. 2000. Salix,
pp. 131-133. In: B. Jonsell, Flora Nordica 1. The
Bergius Foundation, The Royal Swedish Academy of Sciences,
Stockholm.
Buser, R. 1887. Die Brüggerschen
Weidenbastarde, pp.49-92. In: A. Gremli, Neue
Beiträge zur Flora der Schweiz 4. Aarau (cited after Skvortsov 1999).
Buser, R. 1940. Kritische Beiträge zur Kenntnis
der schweizerischen Weiden. Ber. Schweiz. Bot. Ges. 50:
567-788.
Chmelar, J., W. Meusel. 1986. Die Weiden
Europas. Gattung Salix.
Cremer, K.W. 2003. Introduced willows can
become invasive pests in Australia. Biodiversity 4(4):
17-24.
Cutko, A., T. Rawinski. 2007. Flora of
northeastern vernal pools. In: A.J.K. Calhound, P.G.
deMaynadier (eds). Science and conservation of vernal pools in
northeastern North America.
Gleason, H.A., A. Cronquist. 1963. Manual of
vascular plants of northeastern United States and adjacent Canada. D. Van
Nostrand Co., New York. 810 pp.
Gleason, H.A., A. Cronquist. 1991. Manual of
vascular plants of northeastern United States and adjacent Canada. 2nd ed.
The New York Botanical Garden, New York. 910
pp.
Gould, L.L. 2005. The invasive beat: what's
here and where is it? Rhode Island Naturalist 12(2):
13-14.
Haines, A. 2008. Synonymized checklist of New
England tracheophytes. Website http://www.arthurhaines.com/checklist.htm
(accessed 16 January 2009).
Kadis, I. 2007. Unexpected guests become
unwelcome owners. Friends of the Blue Hills Newsletter. 32(1): 1, 5.
Online version: http://www.friendsofthebluehills.org/PDFs/JanMar07.pdf
(accessed 16 January 2009).
Kadis, I., A. Zinovjev. 2008. The alien rusty
willow Salix atrocinerea: A possibility of
hybridization with American willows. Website http://www.salicicola.com/articles/atrocinerea2.
Lagerström, M., T. Uronen. 2005. Pajut
puutarhassa. Küstannusosakeyhtiö Tammi. Helsinki. 216
pp.
Meikle, R.D. 1984. Willows and poplars of Great
Britain and Ireland. Botanical Society of the British Isles Handbook
4.
Rawinski, T. 2005a. European gray willow
(Salix cinerea): A message from Tom Rawinski, USFS.
Invasive Plants Newsbriefs 14, New England Invasive Plant Group [NIPGro],
25 Aug 2005 (cited after Gould 2005).
Rawinski, T. 2005b. In:
Rosenholm, G. Botanist says stealthy invasive willow putting local rare
plants at risk. USDA Forest Service Northeastern Area News Release 14-05.
Website http://www.na.fs.fed.us/nanews/archives/2005/oct05/oct14_05.htm
(accessed 16 January 2009).
Rechinger, H.K. (revised by J.R. Akeroyd).
1993. Salix. In: T.G. Tutin et
al. Flora Europaea, Vol. 1. ed. 2. (Salicaceae edited by J.R.
Edmonson).
Roininen H., J. Tahvanainen, V. Vikberg, and A.
Zinovjev. 2001. Salix aurita—the correct food
plant for Euura cinereae Kopelke 1996
(Hymenoptera,Tenthredinidae). Entomologica Fennica 12:
129-130.
Skvortsov, A. K. 1955. The willows of Central
European Russia and their identification during the wintertime. Bull.
MOIP, otd. biol., LX (3): 115-127. In Russian; English translation at
website http://www.salicicola.com/translations/Skvortsov1955.html.
Skvortsov, A. K. 1968. Ivy SSSR.
Sistematicheskiy i geograficheskiy obzor [Willows of the USSR. Taxonomic
and geographic revision.] Nauka, Moscow. 262 pp.
Skvortsov A. K. 1971. Euro-amerikanische
verwandtschaftliche Beziehungen zweier mediterraner
Salix-Arten. Feddes Repertorium 82(6): 407-420.
English translation at website http://www.salicicola.com/translations/Skv1971EA.html.
Skvortsov, A.K. 1973. [Present distribution and
probable primary range of brittle willow (Salix fragilis L.)], pp. 263-280. In: Problemy
biogeotsenologii, geobotaniki i botanicheskoy geografii. Nauka, Leningrad.
English translation at website http://www.salicicola.com/translations/Skv1973SF.html.
Skvortsov, A.K. 1999. Willows of Russia and
adjacent countries. Taxonomical and geographical revision. Univ. Joensuu.
Faculty of Mathematics and Natural Sciences Report Ser. 39. Joensuu,
Finland. English translation of Skvortsov 1968. Ivy SSSR. Sistematicheskiy
i geograficheskiy obzor [Willows of the USSR. Taxonomic and geographic
revision.] Nauka, Moscow. 262 pp. Online version: http://www.salicicola.com/announcements/skv/skvortsov.pdf.
Tucker, G.C. 1996. Salix atrocinerea—an overlooked willow in New York State. New
York Flora Association Newsletter 7(2): 2.
Tucker, G.C. 2006. Additions to the flora of
Rhode Island. Rhodora 108: 65-71.
Tucker, G.C. 2007. Additions to the flora of
Connecticut. Rhodora 109: 459-463.
Wichura, M. 1865. Die Bastardbefruchtung im
Pflanzenreich, erläutert an den Bastarden der Weiden.
Breslau (cited after Skvortsov 1999).
Zinovjev, A., I. Kadis. 2006. Willows of New
England. Comparison of introduced species Salix atrocinerea and S. cinerea. Website http://www.salicicola.com/notes/atrocinerea_cinerea.
|
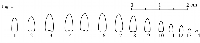 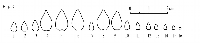                                                        |
||||
|
26 Jan 2009
|
|||||